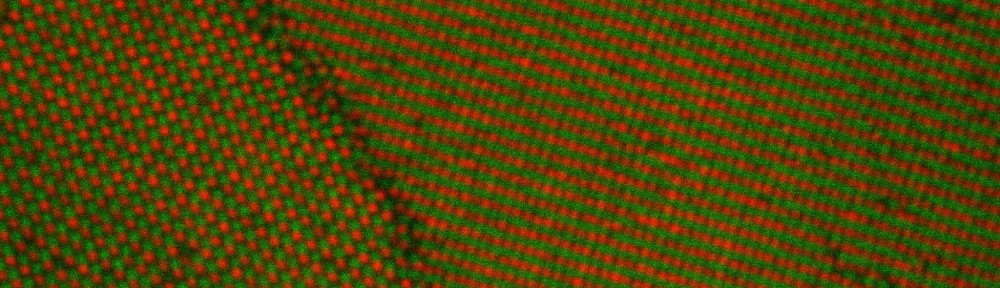
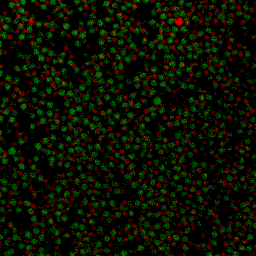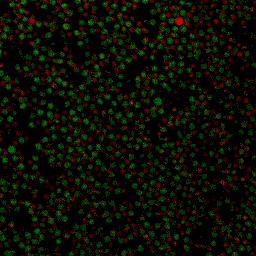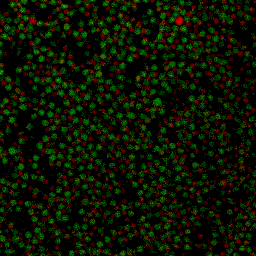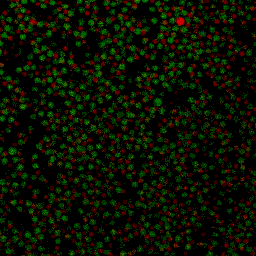
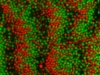
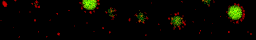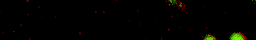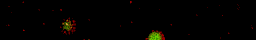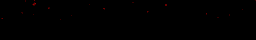During my PhD (2006-2010), we experimentally studied systems of oppositely charged colloidal particles. In our experiments, we used polymethylmethacrylate (PMMA) particles in an oily mixture of the solvents cyclohexylbromide and cis-decahydronaphtalene. Because this solvent mixture is a-polar, ions hardly dissociate and the charge on the colloids is low, typically hundreds of elementary charges per colloid. The charge on particles can e.g. be measured by electrophoresis, i.e. measuring their speed while they are moving in an electric field, or by using optical traps. We mainly investigated what happens when you combine two types of colloids that have an opposite charge after you mix them together, and studied them with confocal laser scanning microscopy. The two particle species were labelled with a different optical dye. Such a dye emits light at a specific range of wavelengths after you shine on it with a laser. The two different dyes respond to distinct laser light frequencies, so the oppositely charged particles can be distinguished, as can be seen in the movie below. The red particles are positively charged, and the green particles are negatively charged.
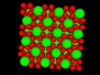
Oppositely charged colloidal particles attract each other and can form so-called “ionic colloidal crystals”, when the interactions become of the order of the thermal energy and the particles efficiently explore configuration space. It can take hours to days for such a crystal to form, starting from a completely mixed dispersion. The type of crystal depends on the size-ratio and the charge-ratio between the two particles.
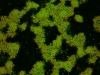
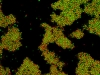
Under some conditions the attractions are initially strong, and the particles get “kinetically trapped” inside a gel. If the attractions become weaker in time, crystals start to grow inside the branches of the gel (see also the confocal microscopy images below).
Gravity also has an effect on the growth of crystals, since it causes the colloids to fall down (sediment) whenever their density is different from the solvent. We studied the effect of minimizing the sedimentation of colloids by slowly rotating our samples on a carousel for various colloidal systems. This way of simulating micro-gravity could in some cases serve as a cheap alternative to expensive experiments using parabolic flights or in the international space station. We compared crystal growth of oppositely charged particles from the fluid and gel phases in the rotating sample (where the sedimentation was time-averaged out) with crystal growth in samples that were stored horizontally and where the oppositely charged colloids effectively sedimented.
When the attractions between the oppositely charged particles are strong, and the size ratio is large, many small positively charged particles can become adsorbed on the surface of one large negatively charged particle. This type of clusters are “overcharged”, which means that the sum of the positive charges on the small particles is higher then the negative charge on the large particle. Therefore, they move to the negative electrode in an electric field. When the field strength is sufficiently large, the small particles “crawl” over the surface of the large particles, and the cluster becomes polarized as can be seen in the movie below. With the field strength is further increased, the oppositely charged particles can even become completely separated (not shown here).
We used our oppositely charged colloids as a model system to study pattern formation out of equilibrium. Since the two particle are oppositely charged they move in opposite directions when the colloids are driven by an electric field. Particles with the same charge line up and form “lanes” in the direction of the field. The movie below starts with a mixed suspension of oppositely charged colloids. Then the electric field is applied in the horizontal direction and lanes form. After a small pause, the field is applied in the other direction (still horizontal) and the lanes form again.
The phenomenon of lane formation is also observed in other systems, such as pedestrians moving in opposite directions (see here, or here). By combining experiments and computer simulations, we studied the mechanism of lane formation for oppositely charged colloids and found that there is a regime of field strengths where the number of particles in a lane increases continuously with the field strength. Particles inside lanes are shielded by the particles in front of them. As a result, they experience fewer head-on collisions than particles outside a lane but do still get little kicks sidewards, which stabilises the lanes.
When the field is periodically reversed at a well-chosen frequency, lanes don’t have the time to fully develop, and the number of head-on collisions per particle increases. As a result, we observed in experiments that the particles form bands that are oriented perpendicular to the field, containing only red or green colloids. We studied the field strengths and frequencies where this band-formation occurs. Below are two movies where you can see the bands form (the movies are faster than in reality). On the left, oppositely charged particles with a diameter of ca. 2.5 micrometer are moving and forming bands in an electric field with strength |E|=17.5 V/mm, and frequency f=0.02 Hz. The electric field is in the horizontal direction.
In the movie on the right, the particles are smaller, ca. 1 micrometer, and the field strength is |E|=104 V/mm. The movie starts with a completely mixed suspension. The bands form when a field of 1.5 Hz is applied. Later, when the frequency is decreased to 0.1 Hz, lanes can once again fully develop, the bands get swept away, and the suspension completely remixes.


Thanks to all people from Soft Condensed Matter Utrecht who collaborated in performing this research.